
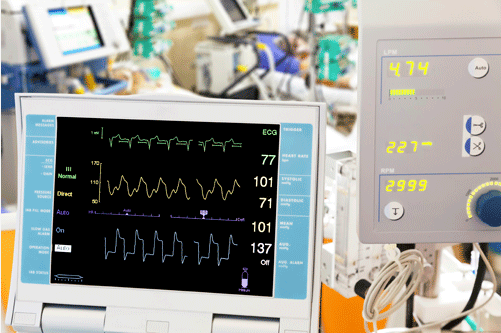
Efficient and high - speed power supplies are used in life - saving and life - enhancing products throughout the medical field. Modern ventilators usually adopt high - speed DC motors to drive the positive pressure in the lungs. These motors require a power supply to start and stop quickly. Understanding motor behavior is crucial for preventing ventilator - related lung injuries. Analysis tools such as the Inverter Motor Drive Analysis (IMDA) software for Tektronix Series 5 and Series 6 oscilloscopes enable you to focus on design by providing automated setup and measurement to simplify testing.
For mobile medical devices, it is necessary to ensure the efficiency of all links in the system. The specifications of electric mobile devices may be relatively strict, so it is necessary to use GaN and SiC technologies. The efficient power supply and IsoVu isolated power probes ensure measurement at the higher end of the power supply in a unique way, thus ensuring that more accurate measurements can be made anywhere in the circuit to diagnose problems.

EMI and EMC compliance is a crucial stage in the development of medical devices. Did you know that 50% of projects cannot pass the EMI/EMC test at one go? A report by Intertek Testing Services points out that due to the failure to apply EMC principles, lack of EMC/EMI knowledge, incorrect application of EMC regulations, unpredictable interactions between circuit components, or the inclusion of non - compliant modules or components in the final product, about half of the products cannot pass the initial EMC test.
Conducting pre - compliance testing greatly increases the success rate of passing the comprehensive regulatory test for the first time, and it doesn't require much effort or time. Through Tektronix spectrum analyzers and SignalVu - PC software, button verification can be performed and settings can be easily made, thus accelerating product launch.

Fiber devices are a type of functional elements with fiber as the basic structural form. They present a fibrous shape, having a slender appearance. The diameter is usually relatively small, and the length can be long or short according to specific design and application requirements. Their shape can be straight, or they can be processed into a style with a certain curvature or flexibility, so as to better adapt to different usage environments and integration requirements.

As more and more healthcare and medical devices are connected in one way or another, the integration of modern connectivity technologies with patient care is increasing day by day. In the new era of the Internet of Medical Things (IoMT), various battery - powered wireless medical devices are becoming more and more common in our daily lives. The categories of traditional and emerging medical devices include fitness wristbands or smartwatches (with the ability of pulse monitoring or heart rate monitoring), blood pressure monitors, pacemakers, pulse oximeters, blood glucose monitors, thermometers, hearing aids. In addition, there are other prototypes under development or new products preparing to be launched before the end of the year.
These types of medical devices share common characteristics. They have low power consumption, are powered by batteries, are small in size, light and flexible, and support wireless connectivity. If these devices suffer from insufficient battery life, in terms of the severity of the consequences, it will bring inconvenience at best, and endanger lives in severe cases. Therefore, in all aspects of the design of electronic consumer medical devices, understanding the power consumption modes and battery life requirements of medical devices are major consideration factors, so as to meet the stringent demands of end - users.
The COVID - 19 pandemic that has swept the globe has left a lasting imprint on the global economy, bringing about permanent changes and has also become a key catalyst for the widespread use of digital healthcare and telemedicine. Amidst this, a “habitual thinking” about digital healthcare is gradually taking shape. With the further strengthening and improvement of the three major scenario elements of policy, market, and data, it means that the rise of digital medical devices has introduced medical technology into a brand - new era. Corresponding challenges have followed closely: To realize digital healthcare and telemedicine, it means that multiple radio frequency devices need to be added to medical equipment to transmit a variety of signals, so that medical staff can conveniently obtain patients' medical information. As is well known, the current frequency spectrum resources are tight, and there are mixed communication signals of various standards in the air. Then how to enable medical equipment with complex radio frequency devices to coexist with other wireless devices without affecting data accuracy?